Cell Therapy Market - Size & Global Industry Analysis
Introspective Market Research, a leading provider of strategic insights across life sciences, today published its deep-dive analysis of the Global Cell Therapy Market . This market encompasses the introduction of viable, modified, or unmodified cells into a patient's body to treat or prevent disease, representing a paradigm shift towards regenerative and curative medicine.
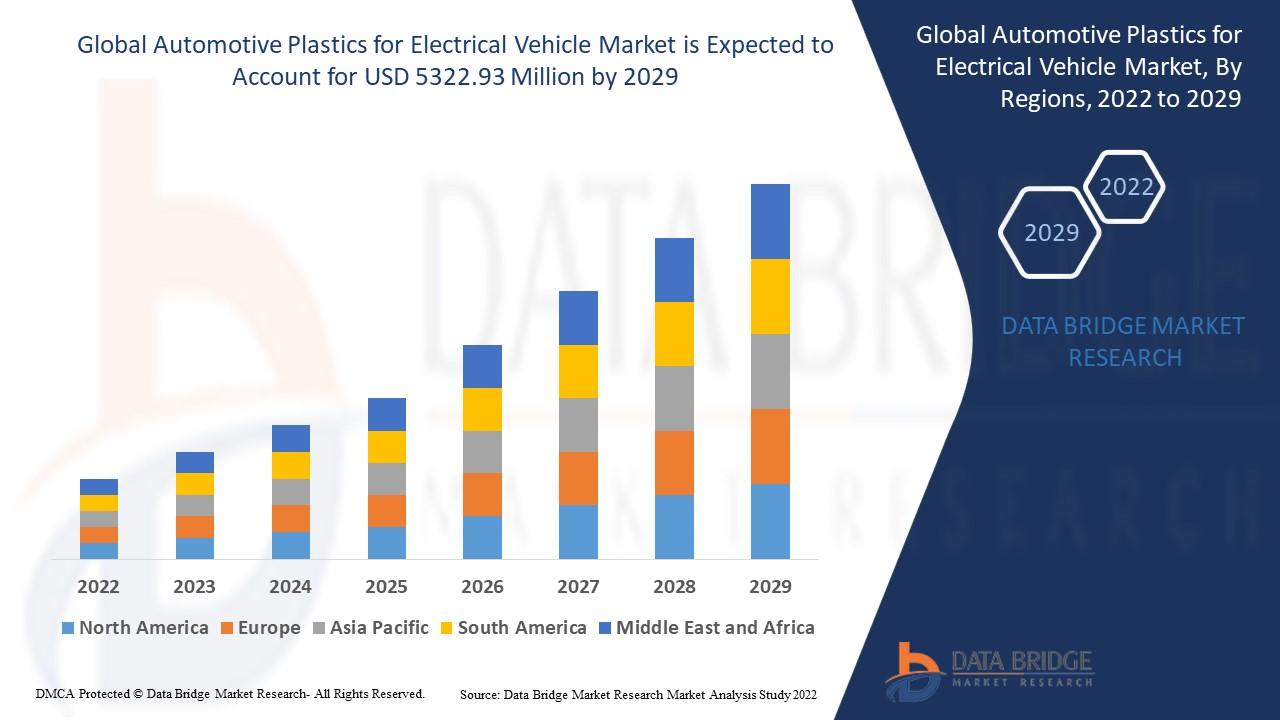
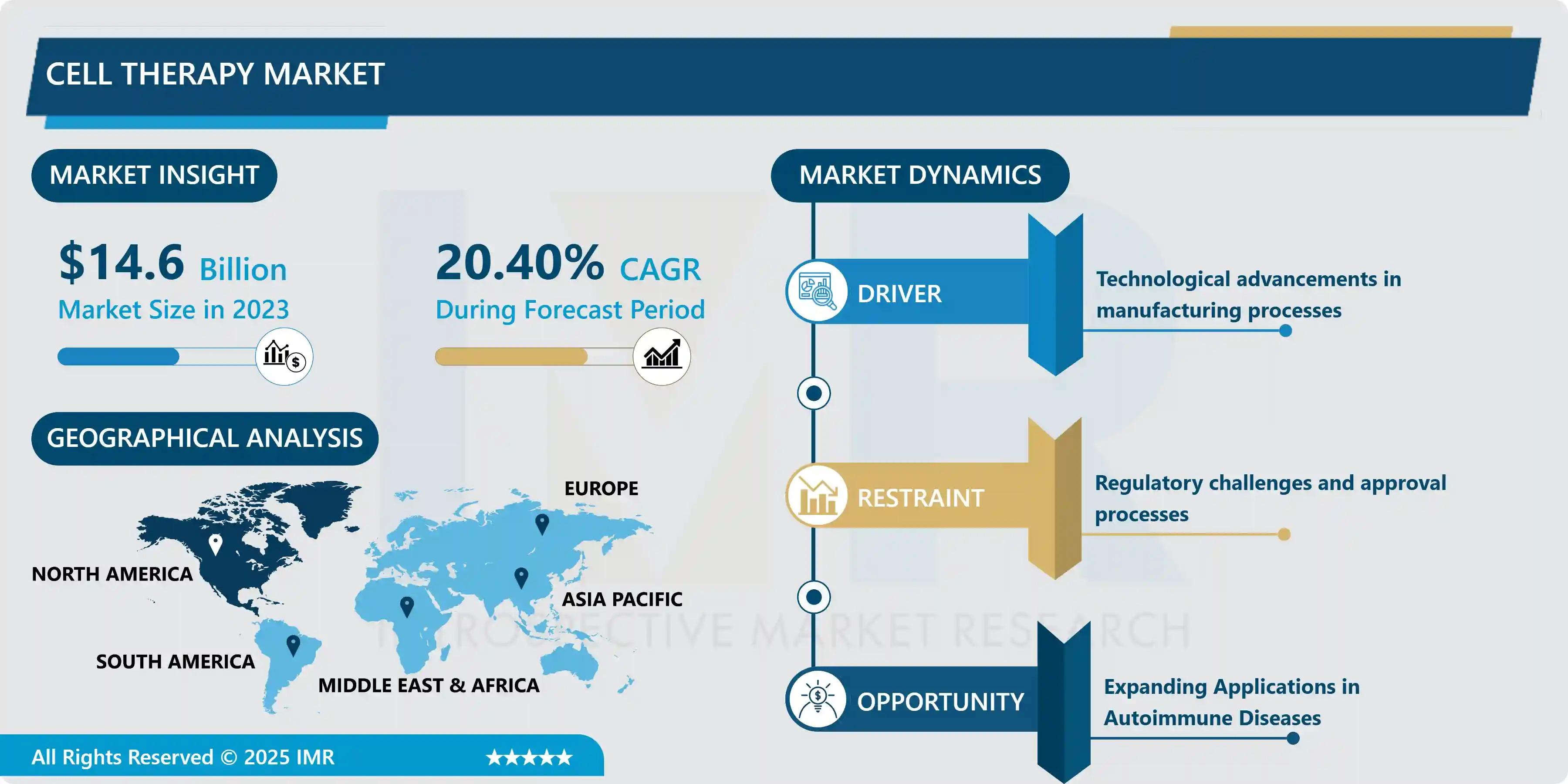
The Global Cell Therapy Market size was valued at USD 14.6 Billion in 2024 and is projected to reach an astounding USD 77.6 Billion by 2032 , recording a phenomenal Compound Annual Growth Rate (CAGR) of 20.4% over the 2024–2032 forecast period. This explosive trajectory is primarily driven by technological advances in manufacturing processes that enhance cell yields and reduce production timelines, alongside the increasing incidence of chronic diseases like cancer and cardiovascular disorders requiring complex cellular interventions.
Quick Insights: Global Cell Therapy Market (2024–2032)
|
Metric |
Insight |
|
2024 Market Valuation |
USD 14.6 Billion |
|
Projected 2032 Valuation |
USD 77.6 Billion |
|
CAGR (2024-2032) |
20.4% |
|
Dominant Therapy Type |
Autologous Therapies (Using the patient's own cells) |
|
Leading End-User |
Hospitals (Primary point of delivery for complex, regulated treatments) |
|
Largest Regional Market |
North America (Held ~40% share in 2023) |
|
Key Market Driver |
Advanced Manufacturing Technologies (Automation and process standardization) |
|
Key Opportunity |
Expanding Applications in Autoimmune Diseases |
Segmentation Spotlight: Autologous Therapies Maintain Lead in Personalized Treatment
The market segmentation highlights the preference for highly personalized treatment modalities:
- By Type: Autologous Therapies (using the patient’s own cells) are expected to dominate the market. This leadership is secured by their inherent safety profile, as they virtually eliminate the risk of immune rejection or Graft-versus-Host Disease (GvHD). Innovations in collection, genetic modification (e.g., in CAR T-Cell therapy), and rapid reinfusion protocols continue to solidify this segment’s appeal. * By End-User: Hospitals command the largest share of the market. They serve as the central point for delivering these complex, highly regulated, and resource-intensive treatments, possessing the necessary infrastructure, specialized clinical staff, and patient management protocols for safe administration.
How is the Focus Shifting to Autoimmunity and Allogeneic Scalability?
The most potent market opportunity lies in the Expanding Applications in Autoimmune Diseases. Researchers are increasingly leveraging cell therapy—particularly regulatory T-cells (Tregs) and mesenchymal stem cells (MSCs)—to rebalance immune responses in conditions like rheumatoid arthritis, multiple sclerosis, and lupus. This represents a significant pivot from merely treating symptoms to modulating the root cause of the immune dysfunction.
Simultaneously, a key industry trend involves the race to develop robust Allogeneic (Off-the-Shelf) Therapies. While autologous therapies are highly effective, they are bespoke, expensive, and time-consuming. The ability to create allogeneic cell lines that can be mass-produced, frozen, and shipped, drastically lowering manufacturing complexity and patient wait times, is attracting massive investment from firms like Novartis AG and Gilead Sciences, Inc.
Expert Insight: Navigating the 'Vein-to-Vein' Logistical Challenge
"The Cell Therapy Market is no longer a question of if the science works, but how we scale the logistics and manufacturing to meet demand affordably," states Dr. Amelia Velez, Principal Consultant at Precedence Research. "The core restraint is the 'vein-to-vein' time—the entire chain from drawing a patient's cells to reinfusing the modified therapeutic product. This process is intensely complex, prone to high variability, and currently incurs immense cost pressures. Future growth depends entirely on harmonizing global regulatory pathways and applying closed-system, fully automated manufacturing platforms to transform this personalized science into a reproducible, scalable industrial process."
Regional Dynamics and Corporate Strategy
North America remains the undeniable market leader, accounting for approximately 40% of the global share in 2023. This dominance is attributed to a highly favorable regulatory climate (including fast-track pathways for regenerative medicine), strong venture capital funding, and the presence of industry heavyweights such as Gilead Sciences (Kite Pharma), bluebird bio, and Celgene.
However, the Asia-Pacific region, driven by advances in China and Japan, is projected to witness the fastest growth. Increased healthcare expenditure, a rapidly aging population, and significant state-sponsored investment in bio-innovation and stem cell research are fueling the establishment of new clinical research sites.
Latest Breakthroughs: Firms are focused on enhancing persistence and reducing immunogenicity. Novartis AG continues to expand its foundational CAR T portfolio, focusing on optimizing cell engraftment. Meanwhile, companies like Astellas Pharma Inc. are pushing into induced Pluripotent Stem Cell (iPSC) derived therapies, aiming to create universal cell lines for broader applications in degenerative diseases.
Challenges: Regulatory Hurdles and High Treatment Costs
The primary restraint impacting the cell therapy sector is the complex web of Regulatory Challenges and Approval Processes. Given the living nature of the treatment, quality control and consistency are exceptionally difficult to maintain, leading to high scrutiny from global health authorities. This regulatory burden directly contributes to the high cost pressures of commercialization, making existing cell therapies prohibitively expensive and hindering broad patient access. Furthermore, scaling production without compromising quality remains a fundamental engineering hurdle.
Case Study: CAR T-Cell Automation for Oncology
A major biopharma manufacturer, addressing the high cost and logistical challenge of manual cell processing for their CAR T-Cell product, integrated a new generation of closed-system, automated manufacturing platforms . This shift reduced the manual labor required by over 70%, simultaneously decreasing the risk of contamination and standardizing the cell modification process. The automation effort, while initially costly, reduced the overall per-dose manufacturing time by 30 days and significantly improved batch-to-batch consistency, paving the way for increased commercial scale and, eventually, lower patient costs.
Call to Action
About Introspective Market Research
Introspective Market Research (IMR) is a trusted provider of comprehensive market intelligence, offering in-depth insights into global industry trends, competitive landscapes, and growth opportunities. Our reports empower businesses to make informed, strategic decisions that accelerate growth and maximize value across diverse sectors.
Contact: Introspective Market Research Phone: +91-74101-03736
Email: sales@introspectivemarketresearch.com
Website: https://introspectivemarketresearch.com/