Healthcare Specialty Enzymes Market: Analysis & Market Share
Introspective Market Research, a leading provider of comprehensive market intelligence, today released its detailed report on the Global Healthcare Specialty Enzymes Market . These specialized enzymes, which act as highly efficient biocatalysts, are moving from niche pharmaceutical components to being fundamental tools in advanced disease detection, therapy development, and regenerative procedures.
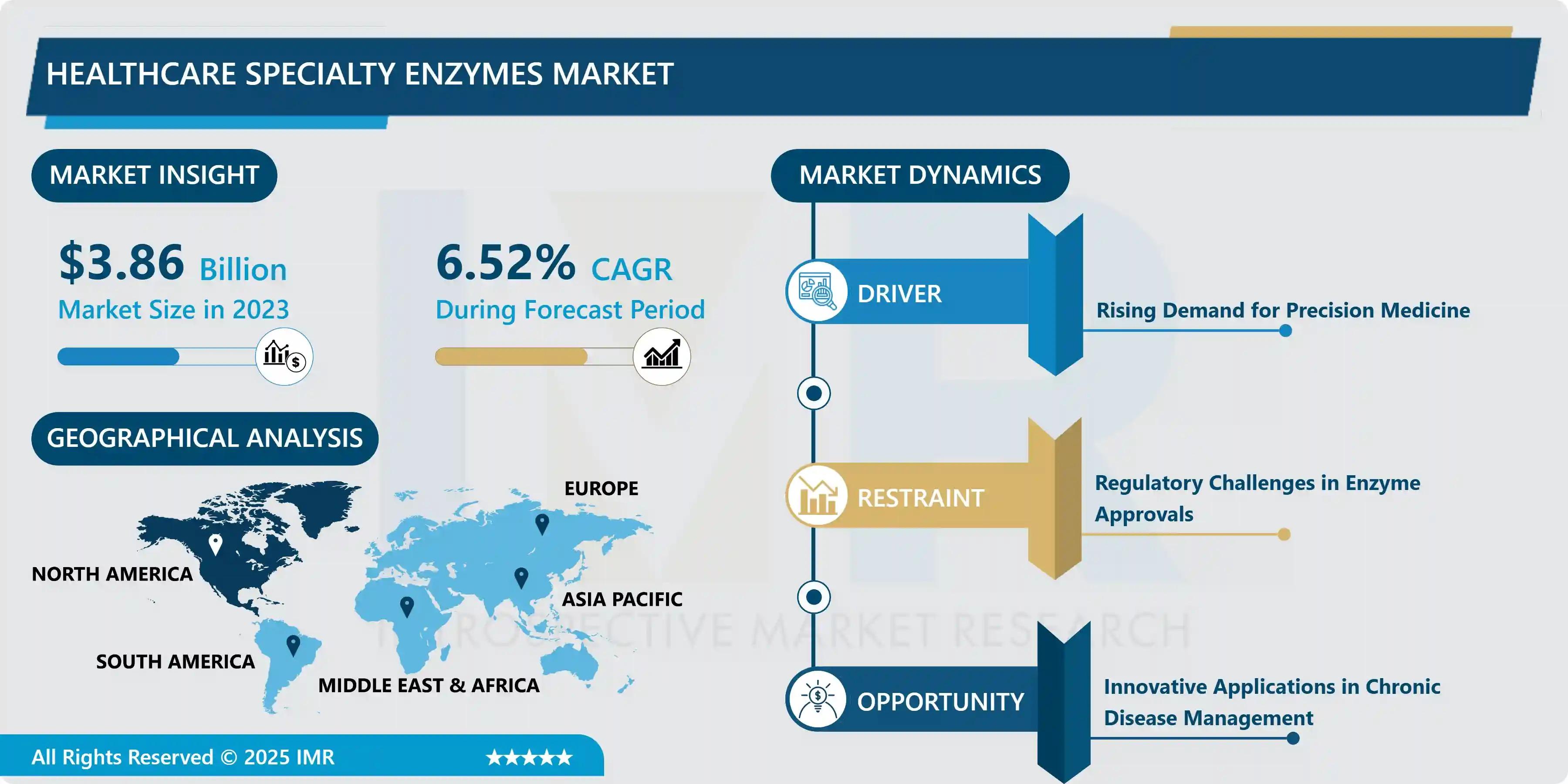
The Healthcare Specialty Enzymes Market size, valued at USD 3.86 Billion in 2024 , is projected to reach USD 6.82 Billion by 2032 , demonstrating a Compound Annual Growth Rate (CAGR) of 6.52% during the forecast period of 2024–2032. This growth is primarily driven by the rising global demand for precision medicine —requiring highly specific enzymatic reagents for accurate diagnostics—and significant capital investment in biopharmaceutical research and development (R&D).
Quick Insights: Global Healthcare Specialty Enzymes Market (2024–2032)
|
Metric |
Insight |
|
2024 Market Valuation |
USD 3.86 Billion |
|
Projected 2032 Valuation |
USD 6.82 Billion |
|
CAGR (2024-2032) |
6.52% |
|
Dominant Application |
Diagnostics (Critical for accurate biomarker detection) |
|
Leading Source |
Microbial (High yield, specificity, and production scalability) |
|
Leading Product Type |
Proteases (Essential for protein processing and therapeutics) |
|
Largest Regional Market |
North America (High R&D expenditure and favorable regulatory framework) |
|
Key Market Opportunity |
Innovative Applications in Chronic Disease Management |
Segmentation Spotlight: Diagnostics Lead the Way to Tailored Treatment
The market’s segmentation highlights its critical clinical utility, particularly in early and accurate intervention:
- By Application: The Diagnostics segment currently commands the largest share of the market. Specialty enzymes are crucial components in various assay kits, enabling the rapid and accurate detection of diseases, monitoring therapeutic drug levels, and identifying specific biomarkers—all essential steps in modern, personalized treatment plans.
- By Source: The Microbial segment (derived from bacteria and fungi) is expected to dominate the market by source. Microbial enzymes are highly favored due to their superior stability, scalability in large-scale fermentation processes, and the ease with which their properties can be engineered for specific medical functions.
- By Product Type: Proteases (enzymes that break down proteins) are the most widely used product type, finding applications in everything from pharmaceutical manufacturing and wound debridement therapies to essential biochemical research.
How are Green Enzymes and AI Reshaping the Biocatalytic Landscape?
A major transformative trend is the industry's shift toward sustainable and ‘green’ enzyme development. Companies are leveraging modern enzyme engineering to create biocatalysts that are highly efficient, require less harsh reaction conditions, and minimize environmental byproducts. This trend aligns not only with global sustainability targets but also with the stringent requirements of pharmaceutical manufacturing.
Furthermore, the integration of Artificial Intelligence (AI) and Machine Learning (ML) is accelerating the discovery and optimization of new enzymes. AI algorithms can analyze vast genetic libraries and predict the structure and function of novel enzymes in a fraction of the time, leading to faster development of highly targeted specialty enzymes for complex diseases. This technological advancement represents a core opportunity to address innovative applications in chronic disease management that currently lack effective solutions.
Expert Insight: The Regulatory Hurdle of Highly Specific Biocatalysts
“Specialty enzymes are the molecular scissors of modern medicine, capable of performing complex biochemical tasks with unparalleled precision,” states Dr. Evelyn Cho, Principal Consultant at Precedence Research. “The growth we are seeing is robust, but it is constantly hampered by the complexity of regulatory approval. Unlike standard chemicals, these biological products are sensitive, often patient-specific, and require rigorous, highly standardized trials to prove efficacy and safety. The industry’s future success lies in establishing uniform global quality standards to ease the regulatory burden without compromising patient safety, especially as these therapies move from diagnostics to direct intervention.”
Regional Dominance and Strategic Corporate Moves
North America continues to hold the dominant market share, bolstered by its highly developed healthcare infrastructure, massive R&D expenditure by biopharmaceutical giants, and a regulatory environment (led by the FDA) that, while strict, offers pathways for innovative drug and diagnostic approvals. The presence of key players like Thermo Fisher Scientific and Abbott Laboratories in the region further cements this leadership.
Meanwhile, emerging markets in Asia-Pacific are showing rapid growth. Increasing healthcare spending, a rising burden of chronic illnesses, and governments actively investing in domestic biotechnology capabilities are expanding the addressable market for specialty enzyme applications across diagnostics and therapeutics.
Corporate Breakthroughs: Companies like Codexis, Inc. and Novozymes are at the forefront of this innovation, utilizing proprietary protein engineering platforms to develop novel enzymes with enhanced stability and activity, specifically for use in high-throughput diagnostic platforms and as advanced biocatalysts in complex drug synthesis.
The Challenges of Regulatory Friction and Operational Cost
The primary market restraint identified is the complex and stringent regulatory approval process for enzyme-based medical products. Since specialty enzymes are sensitive biological materials, any slight variation in manufacturing or source can affect their efficacy and safety, leading to long, expensive approval cycles. This regulatory friction, combined with the inherently high cost of microbial fermentation and purification required for pharmaceutical-grade enzymes, creates a constant upward cost pressure on the final product, potentially restricting adoption in price-sensitive markets.
Case Study: Accelerated Sepsis Detection
Historically, diagnosing sepsis—a time-critical condition—required lengthy blood culture incubation. A diagnostic laboratory implemented a new rapid test utilizing a highly specific microbial protease developed by a key specialty enzyme manufacturer. This enzyme rapidly breaks down specific bacterial cell wall components, releasing a unique biomarker that can be detected in minutes rather than hours. The adoption of this enzyme-based assay resulted in a 75% reduction in time-to-diagnosis, allowing hospitals to initiate life-saving antibiotic treatment significantly sooner and dramatically improving patient outcomes in critical care settings.
Call to Action
Gain Unmatched Intelligence : Download the Full Healthcare Specialty Enzymes Market Report
[ Click Here to Request Sample Report ] (Simulated Link: /reports/healthcare-specialty-enzymes-market/request-sample)
About Introspective Market Research
Introspective Market Research (IMR) is a trusted provider of comprehensive market intelligence, offering in-depth insights into global industry trends, competitive landscapes, and growth opportunities. Our reports empower businesses to make informed, strategic decisions that accelerate growth and maximize value across diverse sectors.
Contact: Introspective Market Research Phone: +91-74101-03736 Email: sales@introspectivemarketresearch.com
Website: https://introspectivemarketresearch.com/
