U.S. Hemostasis Valve Market Forecast: Steady Growth at 5.6% CAGR, USD 116.33 Million by 2034
Market Overview
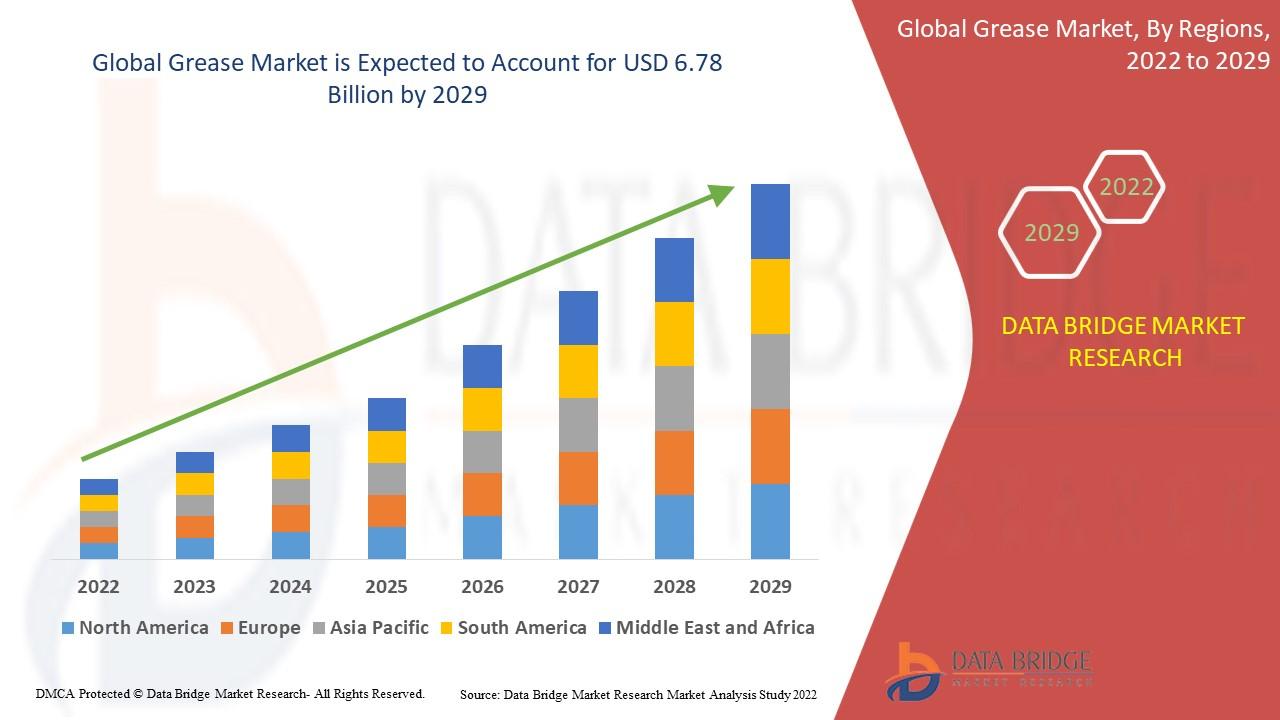
Global U.S. Hemostasis Valve Market size and share is currently valued at USD 67.79 Million in 2024 and is anticipated to generate an estimated revenue of USD 116.33 Million by 2034, according to the latest study by Polaris Market Research. Besides, the report notes that the market exhibits a robust 5.6% Compound Annual Growth Rate (CAGR) over the forecasted timeframe, 2025 - 2034
Hemostasis valves play a critical role in diagnostic and therapeutic interventions such as angioplasty, stent placement, and catheter-based imaging. The increasing prevalence of cardiovascular diseases, coupled with the aging population and growing adoption of minimally invasive surgeries, is driving demand for advanced vascular access solutions.
In the U.S., the healthcare system’s focus on improving procedural safety, patient outcomes, and operational efficiency has accelerated the adoption of hemostasis valves across hospitals, ambulatory surgical centers, and specialty clinics. Continuous product innovations—such as low-profile designs, improved sealing mechanisms, and compatibility with multiple catheter sizes—are contributing to the expansion of this market.
The rise of interventional cardiology and radiology, supported by technological advancements in catheter-based therapies, further underpins market growth. Moreover, the U.S. market benefits from the presence of leading manufacturers and a well-established regulatory framework that encourages quality and innovation in medical devices.
Key Market Growth Drivers
The U.S. Hemostasis Valve Market is propelled by several key factors shaping its development and adoption:
- Rising Burden of Cardiovascular and Peripheral Artery Diseases: Increasing cases of heart disease and vascular disorders are boosting the demand for catheter-based interventions.
- Growing Preference for Minimally Invasive Procedures: Physicians and patients alike are shifting toward less invasive techniques that reduce recovery time and procedural risk.
- Technological Innovations in Valve Design: Development of advanced hemostasis valves with improved sealing efficiency and ease of use enhances procedural safety.
- Expansion of Interventional Radiology Applications: The growing use of catheters in diagnostic and therapeutic imaging expands the utilization of hemostasis valves.
- Increased Healthcare Spending and Infrastructure: Investments in U.S. hospitals and surgical centers are driving higher adoption of interventional devices.
𝐌𝐚𝐣𝐨𝐫 𝐊𝐞𝐲 𝐏𝐥𝐚𝐲𝐞𝐫𝐬:
- Abbott
- Antmed Corporation
- Argon Medical Devices
- B. Braun Melsungen AG
- Boston Scientific Corporation
- Lepu Medical Technology (Beijing) Co.,Ltd.
- Merit Medical Systems
- Nipro
- SCW Medicath Ltd
- Teleflex Incorporated
- TERUMO CORPORATION
𝐄𝐱𝐩𝐥𝐨𝐫𝐞 𝐓𝐡𝐞 𝐂𝐨𝐦𝐩𝐥𝐞𝐭𝐞 𝐂𝐨𝐦𝐩𝐫𝐞𝐡𝐞𝐧𝐬𝐢𝐯𝐞 𝐑𝐞𝐩𝐨𝐫𝐭 𝐇𝐞𝐫𝐞: https://www.polarismarketresearch.com/industry-analysis/us-hemostasis-valve-market
Market Challenges and Opportunities
Despite strong market potential, the hemostasis valve segment faces several challenges and emerging opportunities:
Challenges:
- Stringent Regulatory Approval Processes: Compliance with FDA standards can delay product launches and increase development costs.
- High Cost of Advanced Devices: Premium hemostasis valves with specialized features can be expensive, limiting accessibility in smaller clinics.
- Risk of Device Malfunction or Leakage: Improper sealing can result in complications such as blood loss or air embolism.
- Limited Skilled Professionals: The need for trained interventional cardiologists and radiologists can restrict procedural volume in certain regions.
Opportunities:
- Integration with Advanced Catheter Systems: Development of valves compatible with next-generation catheter designs can enhance procedural outcomes.
- Adoption of Single-Use and Disposable Valves: Growing emphasis on infection control is driving demand for disposable hemostasis valves.
- Expansion in Outpatient and Ambulatory Care Settings: Increasing use of minimally invasive procedures outside hospitals creates new demand channels.
- Technological Advancements in Material Science: Innovations in biocompatible polymers and ergonomic designs are enhancing valve durability and usability.
Market Segmentation
The U.S. Hemostasis Valve Market can be segmented based on product type, application, end user, and distribution channel:
- By Product Type: Y-connector hemostasis valves, one-handed operated valves, double Y-connector valves, and other specialized designs.
- By Application: Cardiology, interventional radiology, peripheral vascular procedures, and neurovascular interventions.
- By End User: Hospitals, ambulatory surgical centers, and specialty clinics.
- By Distribution Channel: Direct sales, medical device distributors, and online platforms.
Regional Insights
Within the United States, regional demand for hemostasis valves varies based on healthcare infrastructure and procedural volume:
- Northeast U.S.: Home to several leading academic hospitals and interventional cardiology centers, driving adoption of premium devices.
- Midwest U.S.: Expanding cardiovascular treatment facilities and increased government healthcare spending support steady market growth.
- Southern U.S.: Rising prevalence of cardiovascular diseases and large patient populations enhance demand for interventional procedures.
- Western U.S.: Technological innovation hubs and major medical device manufacturers contribute to product development and early adoption.
Future Outlook
The future of the U.S. Hemostasis Valve Market looks promising, with continued innovation in device design, materials, and integration capabilities. The increasing trend toward single-use devices is expected to dominate future production, aligning with the growing focus on infection prevention and cost efficiency.
Technological advancements such as hydrophilic coatings, improved torque response, and ergonomic valve configurations will enhance performance and ease of use. Additionally, manufacturers are investing in smart valve systems integrated with sensors for real-time pressure monitoring and feedback, improving procedural precision and safety.
The market will also benefit from the expanding landscape of minimally invasive cardiovascular therapies, including transcatheter valve replacements, electrophysiology studies, and advanced stenting procedures. Furthermore, partnerships between medical device companies and healthcare providers will promote customized product development to meet evolving procedural requirements.
More Trending Latest Reports By Polaris Market Research:
Sickle Cell Disease Treatment Market
Sickle Cell Disease Treatment Market